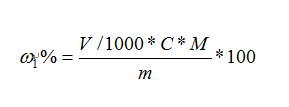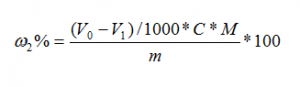1. Determination of Acidity
1.1 Reagents and Solutions
a) Sodium hydroxide standard titration solution: 0.1mol/L.
b) Bromothymol blue indicator: 1g/L (ethanol solution).
1.2 Analysis
Weigh about 1g of the sample (accurate to 0.0002g) and place it in a 250ml Erlenmeyer flask pre-placed with 20ml of water. Add 3-4 drops of bromothymol blue indicator. Titrate with 0.1 mol/L sodium hydroxide standard solution until slightly blue.
1.3 Results
The acidity of glyoxal (in terms of acetic acid) is calculated in terms of mass fraction ω1%, and the number is expressed in %, which is calculated by the following formula:
where:
V——the value of the volume of sodium hydroxide standard titrant consumed by the sample, unit (ml);
C——the exact value of the concentration of sodium hydroxide standard titrant, unit (mol/L);
m——the value of the mass of the sample, in grams (g);
M – the value of the molar mass of acetic acid. The unit is grams per mole (g/mol) (M-60).
1.4 Allowable Error
Take the arithmetic mean of the parallel measurement results as the measurement result. The absolute deviation of parallel determination is not more than 0.05%.
2. Determination of Content
2.1 Method Principle
40% acetaldehyde reacts in potassium hydroxide medium to generate methyl glycolate. Excess potassium hydroxide was neutralized and titrated with a standard hydrochloric acid solution.
2.2 Reagents and Solutions
a) Potassium hydroxide solution: 1.5mol/L.
b) Standard titration solution of hydrochloric acid: 0.5mol/L.
2.3 Analysis
In the conical flask for measuring acidity in 1.1, accurately add 10ml of 1.5mol/L potassium hydroxide solution. Shake well for 15min. Titrate with 0.5mol/L hydrochloric acid standard solution until the solution changes from blue to yellow. At the same time do a blank test.
2.4 Results
The glyoxal content is calculated in mass fraction ω2, and the number is expressed in %, which is calculated by the following formula:
where:
V0——the volume value of the standard titrant of hydrochloric acid consumed in the blank test, in milliliters (ml);
V1——the volume value of the standard titrant of hydrochloric acid consumed in the measurement, the unit is milliliter (ml);
C——The standard value of the concentration of the standard hydrochloric acid titrant, the unit is moles per liter (mol/L);
m——the value of the mass of the sample, in grams (g);
M—the value of the molar mass of glyoxal, in grams per mole (g/mol) (M=58.04).
2.5 Allowable Error
Take the arithmetic mean of the parallel measurement results as the measurement result. The absolute deviation of parallel determination is not more than 0.2%.