Abstract:
Benzoisothiazolinone is an excellent preservative for coatings and plastics. In this paper, the properties, bactericidal mechanism and application fields of benzothiazolinones are described in detail. The advantages of 1,2-benzoisothiazolin-3-one over traditional Kathon fungicides were compared. The synthesis methods of benzoisothiazolinones and the advantages and disadvantages of different synthesis methods are introduced in detail.
Keywords:
Benzisothiazolinone; Preservative; Synthesis; Disulfurized dibenzoic acid.
Main text:
Isothiazolinones have excellent properties such as strong antibacterial ability, small application dose, good compatibility, long duration of efficacy, environmental safety, and wide antibacterial spectrum. These compounds have been receiving high attention. They have been widely used in industrial, agricultural, and pharmaceutical industries.
Among the many isothiazolinones, benzisothiazolinones have excellent alkali and high-temperature resistance. In the field of antisepsis and sterilization, they are gaining attention. Benzisothiazolinones have shown unique advantages in many special industrial fields where conventional anticorrosive products are difficult to be effective.
At present, the use of 1,2-benzisothiazolin-3-one (BIT) in the isothiazolinone fungicides has been second only to 5-chloro-2-methyl-4-isothiazolin-3-one (CMIT) and 2-methyl-4-isothiazolin-3-one (MIT). The position of benzisothiazolinone in the field of fungicides has become more and more important. Based on recent domestic and international research results, the following section reviews the properties and applications of benzoisothiazolinones and analyzes their synthesis methods in detail.
1. Properties of benzoisothiazolinones.
1.1 Properties of benzoisothiazolinones.
Benzisothiazolinones include two major groups of a benzene ring substituted benzisothiazolinones and N-substituted benzisothiazolinones. Most of them have excellent bactericidal properties. This article focuses on N-substituted benzisothiazolinones.
The substituents on the N atom can be straight-chain alkanes, branched alkanes, substituted alkanes, or even other bactericidal groups. When the substituent group on the N atom is an H atom, that is the commonly used BIT.
Except for BIT, the N-substituted benzo isothiazolinone compounds that have been commercialized so far are all straight-chain alkyl substituents. The main ones are N-methyl 1,2-benzisothiazolin-3-one (MBIT), N-butyl 1,2-benzisothiazolin-3-one (BBIT) and N-n-octyl 1,2-benzisothiazolin-3-one (OBIT).
The melting points of the N-substituted benzisothiazolinone compounds decreased with increasing the number of N-substituted carbon atoms.
Among the above four compounds, BIT has the highest melting point, reaching 156-158°C. MBIT is also solid, but its melting point is only 50-55°C. Both BBIT and OBIT are liquids at room temperature.
Benzisothiazolinone compounds are insoluble in water. But BIT can form salts with various inorganic bases and is thus easily soluble in water. Other N-substituted benzisothiazolinone compounds do not form salts and are water-soluble.
The thermal stability of N-substituted benzisothiazolinone compounds increases with the number of N-substituted carbon atoms. Among them, the thermal stability of BIT can reach about 200℃. The thermal stability of BBIT is up to 300°C.
MBIT, BBIT and OBIT have not been widely used due to high prices and poor water solubility. However, they have shown potential applications in the field of antimicrobial agents for plastics. It is only a matter of time before they are developed commercially on a large scale.
1. 2 Properties of 1,2-benzisothiazolin-3-one.
1,2-Benzisothiazolin-3-one (BIT) was first produced on a large scale in the 1980s by the British Imperial Chemical Company. It has excellent antibacterial, anti-mold, and antiseptic properties. 1,2-benzisothiazolin-3-one has outstanding effects on inhibiting the growth of bacteria, mold, yeast, algae, and other microorganisms in organic media. The properties of BIT and the representative minimum inhibitory concentration of BIT are shown in Table 1 and Table 2, respectively.
| Item | Index |
|---|---|
| Appearance | White or light yellow needle-like crystals |
| Melting point | 156~158℃ |
| Molecular weight | 151 |
| Solubility | Soluble in hot water, slightly soluble in some organic solvents, its ammonium salt and sodium salt has high water solubility. |
| Thermal stability | The weight loss started at 180℃ and became obvious at 250℃. |
| Toxicity | Acute oral LD50 in rats: 1400mg/kg. |
| Skin irritation | Guinea pig test: 20% BIT-containing fungicide has no skin sensitivity. |
| Strains spieces | Minimum inhibitory concentration, ppm | Strains spieces | Minimum inhibitory concentration, ppm |
|---|---|---|---|
| E. coli | 30 | Aspergillus niger | 300 |
| Pseudomonas aeruginosa | 200 | Cladosporium herbarum | 100 |
| Staphylococcus aureus | 30 | Moniliformin | 500 |
| Typhoid bacillus | 40 | Penicillin | 150 |
| Proteus vulgaris | 90 | Rhizopus nigricans | 450 |
| Bacillus subtilis | 100 | Brewer’s yeast | 200 |
Note: The active ingredient of BIT is 32%.
As shown in Table 2, BIT has excellent antibacterial activity. The minimum bactericidal activity for E. coli and Staphylococcus aureus, the most common bacteria in daily life, is 30 ppm, and the minimum bactericidal activity for other common bacteria and molds is within 500 ppm.
In addition to excellent bactericidal properties, green is another major characteristic of BIT.
Before the emergence of BIT, the traditional industry mostly used formaldehyde as an anti-mold and anti-bacterial agent. However, formaldehyde is quite toxic and can cause cancer in the nose, mouth, throat, skin, digestive tract and other organs. BIT is undoubtedly the best alternative to formaldehyde.
Toxicological and skin irritation test results show that BIT is less toxic and non-irritating. The acute oral LD50 in rats is 1400 mg/kg, the toxicity classification is low, and the subacute chronic toxicity study did not find any carcinogenicity, teratogenicity, or mutagenicity.
Therefore, Benzisothiazolinone (cas 2634 33 5) can be considered as one of the safe and harmless green products, which is basically harmless to the human body. This allows it to be used not only in high-grade paints and coatings but also in pharmaceutical and food packaging materials to prevent corrosion and mildew.
BIT is also stable over a wide range of pH values and up to 200°C, with no change in bactericidal effect. In addition, BIT is easy to use. BIT degrades rapidly in soil (half-life less than 24 h) and does not accumulate in aquatic organisms.
Currently, the most widely used isothiazolinone fungicide is “Kathon” fungicide. It is a mixture of CMIT and MIT in a ratio of 3:1. “Kathon” fungicides have been widely used in personal care products, detergents, glue, paint, leather, paper and industrial water, oilfield water injection, and other fields.
BIT, CMIT, and MIT are all isothiazolinone derivatives. However, Benzisothiazolinone (BIT) has unique advantages compared with CMIT and MIT (Table 3). This makes BIT has a broader application and commercial prospect than “Kathon”.
| Benzisothiazolinone | Kathon | |
|---|---|---|
| Differences | Chlorine-free, heavy metal-free, stable within pH 3~12, stable below 180°C, compatible with amines. | Some products use Cu2+ as a stabilizer, pH is stable within 3~8.5, stable below 45℃, and will lose activity when encountering amines. |
| Similarities | Do not volatilize, do not release formaldehyde, they are effective against bacteria, mold and yeast, and have low toxicity to higher organisms. | |
As can be seen in Table 3, the thermal stability of BIT is considerably higher than that of “Kathon” (thermal decomposition temperature above 200°C). This makes BIT particularly suitable for the coating process, as it can be added to the system at the beginning of the beating process to provide better protection to the coating system.
In addition, BIT has better alkali resistance than “Kathon”. It is stable in the pH range of 3 to 12. This allows it to be used in alkaline formulations where most preservatives are not available. For example, DBNPA (2,2-dibromo-3-cyanopropionamide), DBDCB (1,2-dibromo-2,4-dicyanobutane), Bro- nopol, and formaldehyde releasing agent type preservatives are not stable in alkaline systems.
Also, due to its excellent stability, BIT provides long-lasting protection for coating products.
2. The bactericidal mechanism of benzoisothiazolinones chemicals.
The bactericidal mechanism of the benzisothiazolinone family of compounds is generally considered to be the same as that of “Kathon”. That is isothiazolinones have a strong penetrating ability to the cell membrane and cell wall of the receptor. After penetrating the cell periphery, they can interact with intracellular sulfur-containing proteins, enzymes or simple molecules to break their S-N bonds. Thus forming S-S bonds with the receptor and disrupting the normal function of the cell.
It is clear that the weaker the S-N bond of an isothiazolinone compound, the better the antibacterial effect.
The relationship between the structure of isothiazolinone compounds and their bactericidal properties has been calculated by quantum chemical methods.
3. Synthesis of benzoisothiazolinones.
3.1 Synthesis route of BIT.
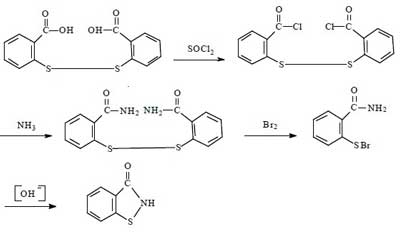
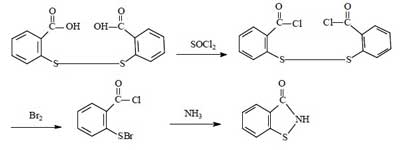
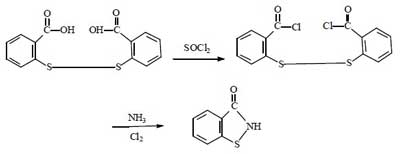
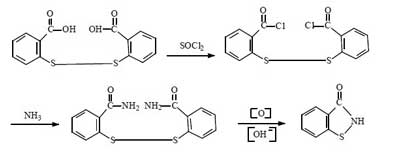
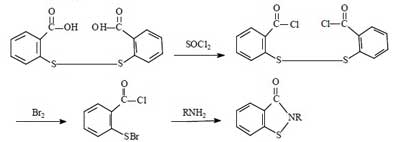
For the synthesis of Benzisothiazolinone, there are four routes in summary. They all use dibenzoic acid disulfide as the starting material, and the reaction mechanism is to chemically break the -S-S- bond of dibenzoic acid disulfide, and then to form BIT by ring formation in the presence of NH3.
Synthesis route 1:
This route has been successful in the laboratory since the 1960s. The total synthetic yield is about 40% to 50%. The advantages of this method are medium synthetic yield and high product purity. The disadvantages are the use of bromine, harsh operating conditions, the need to dry the intermediate dibenzamide disulfide, large investment, the problem of corrosion of equipment is difficult to solve, in addition to the long operating cycle.
Due to the above-mentioned disadvantages of this route, it is usually not used for large-scale industrial production. However, considering that multi-step synthesis can maximize the purity of the product, this method is often applied to the synthesis of Benzisothiazolinone in the laboratory.
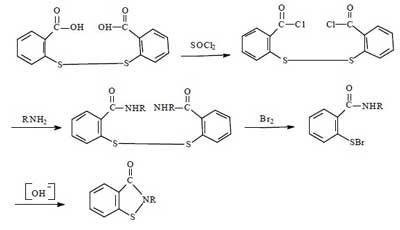
Synthesis route 2:
This route is the most common method for the industrial synthesis of Benzisothiazolinone, and was widely used for the production of BIT until the 21st century, with a total synthetic yield of about 60%-70%. This method is a slight improvement over route 1. The advantages of this method are the high synthetic yield and the relative simplicity of the route. The disadvantages remain the harsh operating conditions, large investment, serious equipment corrosion, and poor product purity.
Synthesis route 3:
This route has a high yield and the reaction process can be carried out continuously, which is also very suitable for industrial production. However, the synthesis process requires the use of chlorine gas. It is more dangerous and difficult to operate than bromine. And the corrosion of equipment is more serious than the above 2 methods. Therefore, this method is not widely used.
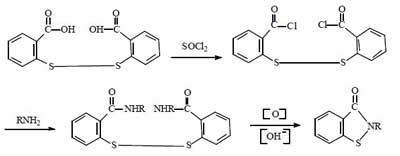
Synthesis route 4:
This route started in the 1990s. The first industrial applications were realized abroad. Now a few domestic companies have successfully applied it in industrial production. The total synthetic yield is about 60%-70%. It is comparable to route 2.
Compared with the above route, this method does not need to use bromine, and the production process is clean and pollution-free. It solves the problem of using halogen in the traditional production method of BIT. The raw material cost is lower and it is suitable for large-scale industrial production. This method is the inevitable way for the future industrial production of BIT.
3. 2 Synthetic routes of N-substituted benzoisothiazolinone derivatives.
There are few reports on the synthesis of N-substituted benzoisothiazolinone derivatives in China and abroad. The reports that we have seen so far are all laboratory synthesis methods. Industrial production has not been reported yet. In summary, there are three synthetic routes for N-substituted benzisothiazolinone derivatives.
Synthesis route 1:
This route has been successfully applied in the laboratory to synthesize N-n-octyl-1,2-benzisothiazolin-3-one. However, this method requires the use of bromine, which poses a high risk of contamination in the production process. In addition, it is corrosive to the equipment, the product purity is poor and the yield is low. All these disadvantages make it unsuitable for application in large-scale industrial production.
Synthesis route 2:
This route has been successfully applied to the laboratory synthesis of N-methyl-1,2-benzisothiazolin-3-one. Due to the stepwise nature of the route, the purity of the synthesized product is high. However, the disadvantages of the route are the use of bromine, the harsh operating conditions, and the corrosion of the equipment. The intermediate dibenzamide disulfide needs to be dried, which leads to a long operation cycle and is difficult to be used in industrial production.
Synthesis route 3:
The advantage of this route is that it does not need to use bromine and the production process is clean and pollution-free. It is also suitable for large-scale industrial production due to the low cost of raw materials.
However, low yields have been reported for this method. Especially for the synthesis of N-substituted derivatives with long molecular chains, the yield is very low. Therefore, further studies are needed to improve the synthesis process and increase the yield.
4. Application of benzoisothiazolinones.
Benzisothiazolinones have the advantages of fast degradation, small application dose, good compatibility and low toxicity due to their good antibacterial ability. They have a very broad application prospect in industrial anti-corrosion, coating industry, pharmaceuticals and pesticides.
4. 1 Coating Industry.
With the increasing awareness of environmental protection and the gradual improvement of environmental regulations, the control of VOCs (volatile organic compounds) and HMPs (hazardous substances) in the coating industry have been further tightened. Various raw materials for coatings, including preservatives, are also developing in the direction of water-based, low toxicity, and environmental protection.
BIT is non-volatile, does not release formaldehyde, does not contain heavy metals and halogens, has low skin irritation, and is biodegradable. It has unique advantages in the anti-corrosion application of environmentally friendly coatings.
BIT not only prevents the spoilage of emulsions and coatings but also makes the coating film resistant to mold attack. For example, by adding 0.5 to 1.0% BIT to interior wall coatings, special anti-mold coatings can be obtained. It is suitable for food factories, cigarette factories, wineries, pharmaceutical factories, hospitals, and other environments that are susceptible to mold attacks.
When producing coatings, BIT can be dissolved in water in any ratio. It is very easy to use. And after coating, BIT’s active ingredient is insoluble in water, so it is not afraid of rain.
4. 2 Industrial corrosion protection.
Benzisothiazolinones have a wide range of applications. They can be found in almost all systems that require corrosion protection in industrial production.
BIT is mainly used in latex products, water-soluble resins, acrylic polymers, polyurethane products, photographic washes, paper, ink, leather, lubricants and other products.
Butylbenzisothiazolinone has been proven to be a good antimicrobial agent for plastic and resin products. It can be used to kill the microorganisms attached to polymer materials. It is very suitable for antibacterial and anticorrosive applications in PU hard foam, soft foam, elastomer, waterproof material, artificial leather, adhesive, etc. It has a very broad application prospect.
In addition, BBIT has good UV resistance and will not fail when used outdoors. It can be mixed with plasticizer, organic carrier, and thinner, which is easy to use. BBIT does not react with additives and does not affect the performance of products, etc.
Methylbenzisothiazolinone and n-octylbenzisothiazolinone have also gained the attention of the industry. Rohm and Haas has applied for a number of patents on methylbenzisothiazolinone and n-octylbenzisothiazolinone as core fungicides. Its related products have also been marketed.
4.3 Pesticides.
So far, many benzisothiazolinone derivatives have been used in herbicides and plant growth regulators, etc.
The 1,2-benzisothiazolin-3-one 1,1-dioxide derivative has some herbicidal activity. It can be used for weed control in sugar beet production. The dosage is 30 g/hm2. It can kill 100% of weeds such as pigweed and chickweed.
In 1990, BIT was found to have high efficiency and broad-spectrum fungicidal properties. It has a significant inhibitory effect on bacteria, fungi and actinomycetes. Therefore, it can have a good effect on the control of plant rots, root rot and early defoliation diseases.
4.4 Pharmaceutical.
Benzisothiazolinones not only have excellent bactericidal effects but also are important pharmaceutical intermediates. Most of their derivatives have good pharmacological effects. In particular, they have antitumor, antiviral and antibacterial effects.
BIT has been found to inhibit platelet aggregation, prevent thrombus formation, anti-atherosclerosis, and prevent heart attack.
Benzisothiazolinone derivatives also have anti-inflammatory, analgesic, and antipyretic properties. Among them, N-substituted benzisothiazolinone derivatives have anti-coagulant and antispasmodic effects. They can be used as platelet aggregation inhibitors. It is an effective drug for the treatment of vascular obstruction diseases.
Benzisothiazolinones have a relatively short history of application in medicine. Large-scale industrial production has not been seen in China. Therefore, there are great prospects for research and development.
5. Conclusion.
Benzisothiazolinones, as an important industrial bactericidal and preservative agent, have been widely used in many applications.
Further studies on the bactericidal mechanism and synthesis process, as well as the development of novel benzisothiazolinone derivatives, are the future research priorities in this field.
At present, the synthesis of benzisothiazolinones has made great progress. The industrialization has been achieved. However, how to further improve the yield, especially the synthesis yield of N-substituted benzisothiazolinone derivatives, and reduce the amount of three wastes in the industrial production process still need more in-depth research.